Marco Rossi was looking forward to his rookie season in the National Hockey League. The 19-year-old prospect was the top point scorer among major junior ice hockey players in the 2019–20 season. Now he was set to impress with the Minnesota Wild. The team had selected Rossi ninth overall in the 2020 league draft ahead of the pandemic-shortened season of the North American league that kicked off in January. However, Rossi’s professional debut was not to be.
At pre-season training camp, Rossi failed his medical examination. A routine cardiac test revealed inflammation around the heart muscles, a condition known as myocarditis. If Rossi continued to skate, his heart might suddenly stop beating, and he could die. Although he felt well, it seemed that Rossi—or at least his heart—had not yet fully recovered from COVID-19, which he had contracted two months earlier.
Rossi flew to his home town in Austria to recuperate. He would miss the entire 56-game season and subsequent play-offs. The young star was bitterly disappointed. “He worked so hard for so many months and he was ready to come out with a bang,” says Serge Payer, Rossi’s agent and a former league player. For Rossi, this “was very much unexpected”, Payer says. It also surprised many cardiologists.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Other viruses are known to infect the heart and spur inflammation. Coxsackieviruses, for example, are a major cause of myocarditis and other heart-muscle defects. When viral pathogens such as these strike, people usually develop chest pains, shortness of breath or some other overt signs of illness. SARS-CoV-2, the virus responsible for COVID-19, is different. Not only do few people diagnosed with coronavirus-induced myocarditis complain of cardiac issues, but they can also have few or no symptoms of infection whatsoever.
Raul Mitrani is a cardiac electrophysiologist at the University of Miami Miller School of Medicine in Florida. Last year, Mitrani and his colleagues gave a name to the constellation of heart-related problems observed among people recovering from COVID-19: post-COVID-19 cardiac syndrome1. “There are a lot of unknowns still,” Mitrani says. “But what we’re ultimately worried about is heart decompensation and dangerous arrhythmias.” The former involves a sudden worsening of heart failure; the latter is an uneven heartbeat. Both can trigger sudden cardiac death.
Scientists have begun to study the phenomenon in the lab, exposing heart tissue derived from stem cells to SARS-CoV-2 and chronicling the damage inflicted. And clinicians are continuing to track people who have had COVID-19 to better understand the long-term cardiac risks. Although it’s too soon to make definitive conclusions, the extent of cardiac injury and inflammation observed using magnetic resonance imaging (MRI)—the most definitive and comprehensive tool for diagnosing myocarditis—has put the field on high alert.
“We need to understand more about what these MRI abnormalities mean,” says Saurabh Rajpal, a cardiologist at the Ohio State University Wexner Medical Center in Columbus.
Taken to heart
From the earliest days of the COVID-19 pandemic, it was clear that the coronavirus was wreaking havoc on the heart. Initial reports described people with worryingly high levels of the protein troponin in their blood, an indicator of cardiac injury.
Acute heart failure, arrhythmias and blood clots are all problems for people hospitalized with COVID-19. Autopsies frequently show signs of the virus’s genetic material inside cardiac tissue, a consequence of the fact that the receptor through which SARS-CoV-2 invades lung cells is also found in abundance in heart tissue.
Researchers soon found out that the heart-wrecking effects of COVID-19 are not limited to people with symptoms, or even to people with active infections. Last July, researchers described2 abnormal imaging findings on heart scans taken from people who had recently had COVID-19, some of whom were asymptomatic. Of the 100 people studied, 78 had some kind of heart irregularity around two months after infection—and 60 showed signs of ongoing myocardial inflammation.
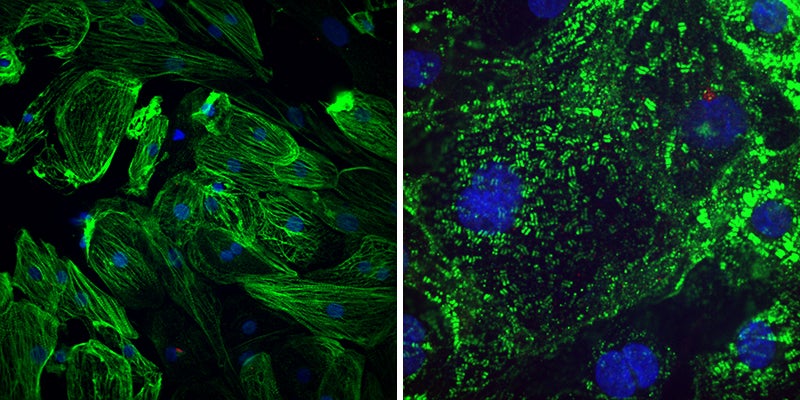
Healthy heart muscle (left) have long fibres that allow them to contract. The virus SARS-CoV-2 causes these fibres to break apart (right), which might explain the lasting cardiac effects seen in people who have had COVID-19. Credit: Gladstone Institutes
“That was quite worrisome and created quite a stir,” Mitrani says. In particular, the idea that COVID-19 could stealthily inflict sustained damage on the heart raised alarm bells among the sports-medicine community, given the particularly grave risk that myocarditis poses for athletes. Citing “potential serious cardiac side effects”, last August, several US university leagues temporarily put their seasons on hold.
Typically, if athletes are diagnosed with myocarditis, they are taken out of play for at least three months to give the heart a chance to heal. But that decision is usually made after people show outward signs of disease. Physicians were unsure what to advise athletes with subclinical myocarditis after COVID-19, which is detectable only with an MRI scanner.
An initial report from Rajpal and his colleagues seemed to confirm the fears of many. A study of 26 university athletes who had tested positive for COVID-19 found signs of prolonged heart inflammation in 4 individuals, all of whom had been asymptomatic or had had mild disease3. That study did not include a non-athletic group for comparison, however. And clinicians such as Scott Reeder, a radiologist at the University of Wisconsin–Madison, were quick to point out that, in some young and healthy athletes, imaging abnormalities suggestive of myocarditis might simply reflect changes that occur in the heart because of high endurance training. “Athletic remodelling is a potential confounder,” Reeder says. Rajpal also acknowledges the limitations of the initial study.
Reeder and others have performed more-controlled analyses on thousands of athletes, both professional4 and amateur5–7. Collectively, they have revised the estimate downwards, and persistent myocarditis is now thought to affect between 1% and 5% of elite athletes who contract COVID-19—an incidence low enough that most sports bodies no longer feel the need to cancel or postpone events over cardiac concerns, but high enough for them to take into consideration the safety of individual players.
Diagnostic uncertainty
Many sports organizations require athletes diagnosed with COVID-19 to undergo a battery of cardiac tests before they are allowed to return to competition. For example, all the major North American professional sports leagues that resumed competition during the pandemic mandated that athletes recovering from COVID-19 have troponin blood tests, electrocardiograms (which measure the heart’s electrical activity) and echocardiograms (which measure the heart’s structure and function), followed by an MRI and other tests if anything suspicious is revealed.
These screening protocols were imposed early in the pandemic because of an abundance of caution. But in light of emerging data, specialists are debating whether they are the prudent choice to save lives or represent diagnostic overkill. If players such as Marco Rossi are outliers, does it make sense to do all these tests routinely on every athlete who contracts SARS-CoV-2 but remains asymptomatic?
Cardiac MRI, for instance, is an expensive procedure, with results that can be complicated by heart remodelling. And because of the low diagnostic yield, a sports programme might have to spend upwards of US$500,000 on scans to catch a single case of the condition.
Of course, those rare diagnoses could make all the difference to athletes such as Rossi. Several top athletes, including a university football player in the United States and a professional basketball player in Serbia, have died from heart complications after contracting COVID-19, and it’s conceivable that an earlier diagnosis of myocarditis could have kept those individuals off the field or the court.
For non-athletes who recover from COVID-19, most heart specialists agree that full cardiovascular work-ups are unnecessary—and clinical evidence suggests that most people who don’t experience cardiac complications after infection have little to worry about, at least in the short term. Luis Ortega Paz, an interventional cardiologist at the Hospital Clinic of Barcelona in Spain, has spent months tracking hundreds of people who have recovered from COVID-19 and he has seen no uptick in the incidence of cardiovascular consequences such as heart attacks or strokes. “Now the focus should be the long-term outcomes,” Ortega Paz says, “so we can get a complete picture of the disease.”
Bruce Conklin, a stem-cell researcher at the Gladstone Institutes in San Francisco, California, agrees. His father developed scarlet fever as a child, before the advent of antibiotics to treat the bacterial illness. The disease caused scarring inside his father’s heart, necessitating valve-replacement surgery decades later. Conklin wonders whether COVID-19 could have similar consequences years from now. “You do worry long term if there’s going to be a problem,” he says.
That’s why researchers at Gladstone and elsewhere have turned to tissue-engineered heart models—both to study the molecular consequences of SARS-CoV-2 infections and, ideally, to find ways of halting or reversing the damage.
Breach of contractile
Conklin’s team, for example, coaxed reprogrammed stem cells, derived from the skin of healthy donors, to form cardiac muscle cells—and found that the virus attacked these cells with gusto. “The infectivity was really quite stunning,” he says.
The infected heart muscle cells had a series of genetic and structural defects in their contractile machinery8. Under the microscope, the researchers could see that contractile filaments known as sarcomeres were all sliced up. “Similar to what one would imagine if cutting a loaf of bread for sandwiches,” Conklin says.
The findings dovetail with another report describing a heart model, derived from stem cells, of SARS-CoV-2 infection9. After exposure to the virus, the mini-hearts struggled to beat properly, their sarcomeres were in disarray, and the infected cells released copious amounts of cytokines, a type of inflammatory distress signal, before eventually dying off.
Rossi is hopeful that his heart tissue hasn’t incurred that type of irreparable damage. He has continued to rest and undergo regular medical evaluations and, like all National Hockey League players, he is excited about competing in front of fans again when the next, more normal, hockey season begins in October.
In a tweet in February, he wrote: “Like many, I was originally shocked and very disappointed, yet at this time I am very optimistic that my health is and will be good to return to train/play.” By then, the pandemic will hopefully be in the rear-view mirror and future sports stars won’t suffer the same fate.
This article is part of Nature Outlook: Heart health, an editorially independent supplement produced with the financial support of third parties. About this content.
References
Mitrani, R. D., Dabas, N. & Goldberger, J. J. Heart Rhythm 17, 1984–1990 (2020).
Puntmann, V. O. et al. JAMA Cardiol. 5, 1265–1273 (2020).
Rajpal, S. et al. JAMA Cardiol. 6, 116–118 (2021).
Martinez, M. W. et al. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2021.0565 (2021)
Starekova, J. et al. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.7444 (2021).
Clark, D. E. et al. Circulation 143, 609–612 (2021).
Hwang, C. E. et al. Preprint at medRxiv https://doi.org/10.1101/2021.01.07.21249407 (2021).
Perez-Bermejo, J. A. et al. Sci. Transl. Med 13, eabf7872 (2021).
Bailey, A. L. et al. JACC Basic Transl. Sci. 6, 331–345 (2021).
