As the world sped towards a pandemic in early 2020, evolutionary biologist Jesse Bloom gazed into the future of SARS-CoV-2. Like many virus specialists at the time, he predicted that the new pathogen would not be eradicated. Rather, it would become endemic—the fifth coronavirus to permanently establish itself in humans, alongside four ‘seasonal’ coronaviruses that cause relatively mild colds and have been circulating in humans for decades or more.
Bloom, who is based at the Fred Hutchinson Cancer Research Center in Seattle, Washington, saw these seasonal coronaviruses as potentially providing a roadmap for how SARS-CoV-2 might evolve and for the future of the pandemic. But little is known about how these other viruses continue to thrive. One of the best-studied examples—a seasonal coronavirus called 229E—infects people repeatedly throughout their lives. But it’s not clear whether these reinfections are the result of fading immune responses in their human hosts or whether changes in the virus help it to dodge immunity. To find out, Bloom got hold of decades-old blood samples from people probably exposed to 229E, and tested them for antibodies against different versions of the virus going back to the 1980s.
The results were striking. Blood samples from the 1980s contained high levels of infection-blocking antibodies against a 1984 version of 229E. But they had much less capacity to neutralize a 1990s version of the virus. They were even less effective against 229E variants from the 2000s and 2010s. The same held true for blood samples from the 1990s: people had immunity to viruses from the recent past, but not to those from the future, suggesting that the virus was evolving to evade immunity.
“Now that we’ve had almost two years to see how SARS-CoV-2 evolves, I think there are clear parallels with 229E,” says Bloom. Variants such as Omicron and Delta carry mutations that blunt the potency of antibodies raised against past versions of SARS-CoV-2. And the forces propelling this ‘antigenic change’ are likely to grow stronger as most of the planet gains immunity to the virus through infection, vaccination or both. Researchers are racing to characterize the highly mutated Omicron variant. But its rapid rise in South Africa suggests that it has already found a way to dodge human immunity.
How SARS-CoV-2 evolves over the next several months and years will determine what the end of this global crisis looks like—whether the virus morphs into another common cold or into something more threatening such as influenza or worse. A global vaccination push that has delivered nearly 8 billion doses is shifting the evolutionary landscape, and it’s not clear how the virus will meet this challenge. Meanwhile, as some countries lift restrictions to control viral spread, opportunities increase for SARS-CoV-2 to make significant evolutionary leaps.
Scientists are searching for ways to predict the virus’s next moves, looking to other pathogens for clues. They are tracking the effects of the mutations in the variants that have arisen so far, while watching out for new ones. They expect SARS-CoV-2 eventually to evolve more predictably and become like other respiratory viruses—but when this shift will occur, and which infection it might resemble is not clear.
Researchers are learning as they go, says Andrew Rambaut, an evolutionary biologist at the University of Edinburgh, UK. “We haven’t had much to go on.”
An early plateau
Scientists tracking the evolution of SARS-CoV-2 are looking out for two broad categories of changes to the virus. One makes it more infectious or transmissible, for instance by replicating more quickly so that it spreads more easily through coughs, sneezes and wheezes. The other enables it to overcome a host’s immune response. When a virus first starts spreading in a new host, the lack of pre-existing immunity means that there is little advantage to be gained by evading immunity. So, the first—and biggest—gains a new virus will make tend to come through enhancements to infectivity or transmissibility.
“I was thoroughly expecting that this new coronavirus would adapt to humans in a meaningful way—and that would probably mean increased transmissibility,” says Wendy Barclay, a virologist at Imperial College London.
Genome sequencing early in the pandemic showed the virus diversifying and picking up about two single-letter mutations per month. This rate of change is about half that of influenza and one-quarter that of HIV, thanks to an error-correcting enzyme coronaviruses possess that is rare among other RNA viruses. But few of these early changes seemed to have any effect on the behaviour of SARS-CoV-2, or show signs of being favoured under natural selection.
An early mutation called D614G within the gene encoding the virus’s spike protein—the protein responsible for recognizing and penetrating host cells—seemed to offer a slight transmissibility boost. But this gain was nothing like the leaps in transmissibility that researchers would later observe with the variants Delta and Alpha, says Sarah Otto, an evolutionary biologist at the University of British Columbia in Vancouver, Canada.
Otto sees the virus’s evolution as like walking in a landscape, where higher elevations equate to improved transmissibility. The way she sees it, when SARS-CoV-2 began spreading in humans it seemed to be on a ‘fitness plateau’ surrounded by a landscape of many possible evolutionary outcomes. In any given infection, there were probably thousands of viral particles each with unique single-letter mutations, but Otto suspects that few, if any, of these made the virus more infectious. Most changes probably reduced transmissibility.
“If the virus entered at a reasonably high point, any one-step mutation would take it downhill,” Otto says. Summiting higher peaks required the combinations of several mutations to make more-significant gains in its ability to spread.
Reaching new heights
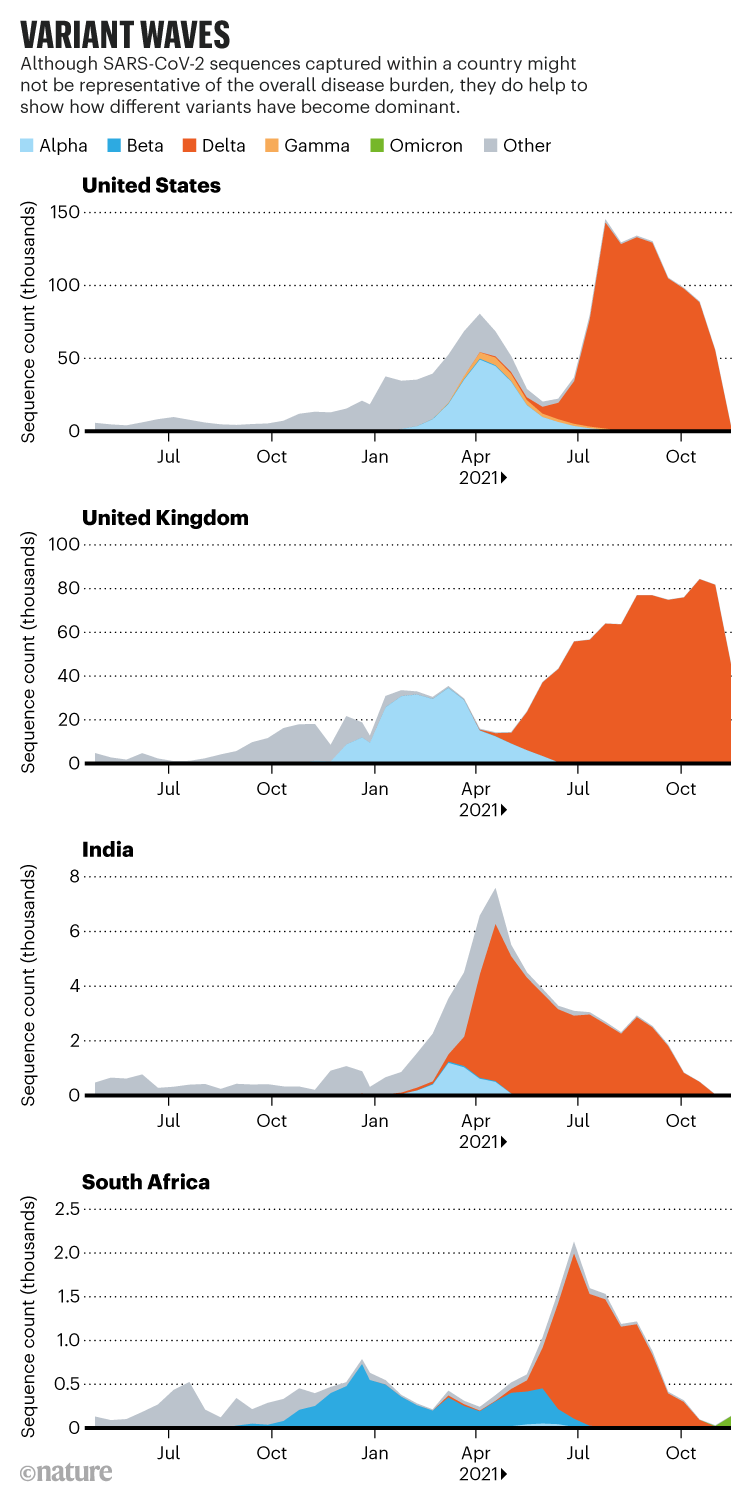
In late 2020 and early 2021, there were signs that SARS-CoV-2 had scaled some distant peaks. Researchers in the United Kingdom spotted a variant called B.1.1.7 that contained numerous mutations in its spike protein. “It was a bit unusual because it seemed to come out of nowhere,” says Francois Balloux, a computational biologist at University College London.
That variant—since renamed Alpha—spread at least 50% faster than earlier circulating lineages. UK public-health officials linked it to a mysterious rise in cases in southeast England during a national lockdown in November 2020. Around the same time, virus hunters in South Africa linked another mutation-laden variant called B.1.351—now known as Beta—to a second wave of infections there. Not long after, a highly transmissible variant, now called Gamma, was tracked to Amazonas state in Brazil.
These three ‘variants of concern’ share some mutations, particularly in key regions of the spike protein involved in recognizing the host-cell ACE2 receptors that the virus uses to enter cells. They also carried mutations similar or identical to those spotted in SARS-CoV-2 in people with compromised immune systems whose infections lasted for months. This led researchers to speculate that long-term infections might allow the virus to explore different combinations of mutations to find ones that are successful. Typical infections lasting days offer fewer opportunities. Super-spreading events, where large numbers of people are infected, might also explain why some variants flourished and others fizzled out.
Whatever their origins, all three variants seemed to be more infectious than the strains they displaced. But Beta and Gamma also contained mutations that blunted the potency of infection-blocking ‘neutralizing’ antibodies triggered by previous infection or vaccination. This raised the possibility that the virus was beginning to behave in the ways predicted by Bloom’s studies of 229E.
The three variants spread around the world, particularly Alpha, which sparked new waves of COVID-19 as it came to dominate in Europe, North America, the Middle East and beyond. Many researchers expected that a descendant of Alpha—which seemed to be the most infectious of the bunch—would pick up additional mutations, such as those that evade immune responses, to make it even more successful. “That absolutely proved not to be the case,” says Paul Bieniasz, a virologist at Rockefeller University in New York City. “Delta came out of left field.”
Credit: Nature doi: https://doi.org/10.1038/d41586-021-03619-8; Source: Covariants.org
The Delta dilemma
The Delta variant was identified in India’s Maharashtra state during a ferocious wave of COVID-19 that hit the country in the spring of 2021, and researchers are still taking stock of its consequences for the pandemic. Once it arrived in the United Kingdom, the variant spread quickly and epidemiologists determined that it was about 60% more transmissible than Alpha, making it several times as infectious as the first circulating strains of SARS-CoV-2. “Delta is kind of a super-Alpha,” says Barclay. “I think the virus is still looking for solutions to adapt to the human host.”
Studies from Barclay’s laboratory and others suggest that Delta made significant gains in its fitness by improving its ability to infect human cells and spread between people. Compared with other variants, including Alpha, Delta multiplies faster and to higher levels in the airways of infected individuals, potentially outpacing initial immune responses against the virus.
Yet researchers expect such gains to become ever smaller. Scientists measure a virus’s inherent ability to spread in an immunologically naive population (that is, unvaccinated and not exposed to the virus previously) by a number called R0, which is the average number of people an infected person infects. Since the start of the pandemic this figure has jumped as much as threefold. “At some point, I would expect that increased transmissibility will stop happening,” says Bloom. “It’s not going to become infinitely transmissible.” Delta’s R0 is higher than seasonal coronaviruses and influenza, but still lower than that of polio or measles.
Other established human viruses do not make the leaps in infectivity that SARS-CoV-2 has in the past two years, and Bloom and other scientists expect the virus to eventually behave in the same way. Trevor Bedford, an evolutionary biologist at the Fred Hutchinson, says the virus must balance its ability to replicate to high levels in people’s airways with the need to keep them healthy enough to infect new hosts. “The virus doesn’t want to put someone in bed and make them sick enough that they’re not encountering a number of other people,” he says. One way for the virus to thread this needle would be to evolve to grow to lower levels in people’s airways, but maintain infections for a longer period of time, increasing the number of new hosts exposed to the virus, says Rambaut. “Ultimately there’s going to be trade-off between how much virus you can produce and how quickly you elicit the immune system.” By lying low, SARS-CoV-2 could ensure its continued spread.
If the virus evolved in this way, it might become less severe, but that outcome is far from certain. “There’s this assumption that something more transmissible becomes less virulent. I don’t think that’s the position we should take,” says Balloux. Variants including Alpha, Beta and Delta have been linked to heightened rates of hospitalization and death—potentially because they grow to such high levels in people’s airways. The assertion that viruses evolve to become milder “is a bit of a myth”, says Rambaut. “The reality is far more complex.”
The rise of Omicron
Delta and its descendants now account for the vast majority of COVID-19 cases worldwide. Most researchers expected these Delta lineages to eventually outcompete the last holdouts. But Omicron has undermined those predictions. “A lot of us were expecting the next weird variant to be a child of Delta, and this is a bit of a wild card,” says Aris Katzourakis, a specialist in viral evolution at the University of Oxford, UK. Teams in Botswana and South Africa identified the variant in late November—although researchers say it is unlikely to have originated in either country—and health officials have linked it to a rapidly growing outbreak centred in South Africa’s Gauteng province. The variant harbours around 30 changes to spike, many shared with the other variants of concern, and scientists worldwide are working to gauge the threat it poses.
The swift rise in cases of Omicron in South Africa suggests that the new variant has a fitness advantage over Delta, says Tom Wenseleers, an evolutionary biologist and biostatistician at the Catholic University of Leuven in Belgium. Omicron carries some of the mutations associated with Delta’s sky-high infectivity. But if increased infectivity were the sole reason for its rapid growth, it would translate to an R0 in the 30s, Wenseleers says. “That’s very implausible.”
Instead, he and other researchers suspect that Omicron’s rise may be largely due to its ability to infect people who are immune to Delta through vaccination or previous infection.
Scientists’ portrait of Omicron is still blurry and it will take weeks before they can fully assess its properties. But if the variant is spreading, in part, because of its ability to evade immunity, it fits in with theoretical predictions about how SARS-CoV-2 is likely to evolve, says Sarah Cobey, an evolutionary biologist at the University of Chicago in Illinois.
As gains in SARS-CoV-2’s infectivity start to slow, the virus will have to maintain its fitness through overcoming immune responses, says Cobey. For instance, if a mutation or set of mutations halved a vaccine’s ability to block transmission, this could vastly increase the number of available hosts in a population. Cobey says it’s hard to imagine that any future gains in infectivity could provide the same boost.
That evolutionary path, towards immune evasion and away from gains in infectivity, is common among established respiratory viruses such as influenza says Adam Kucharski, a mathematical epidemiologist at the London School of Hygiene and Tropical Medicine. “The easiest way for the virus to cause new epidemics is to evade immunity over time. That’s similar to what we see with the seasonal coronaviruses.”
Lab experiments and sequencing of circulating variants have identified a smorgasbord of mutations in the spike protein that weaken the potency of neutralizing antibodies triggered by infection and vaccination. Variants carrying these mutations, such as Beta, have blunted the effectiveness of vaccines. But they have not obliterated the protection that the shots offer, particularly against severe disease.
Compared with other variants, Omicron contains many more of these mutations, particularly in the region of spike that recognizes host cells. Preliminary analysis from Bloom suggests that these mutations might render some portions of spike unrecognizable to the antibodies raised by vaccines and previous infection with other strains. But lab experiments and epidemiological studies will be needed to fully appreciate the effects of these mutations.
Evolving to evade immune responses such as antibodies could also carry some evolutionary costs. A spike mutation that dodges antibodies might reduce the virus’s ability to recognize and bind to host cells. The receptor-binding region of spike—the major target for neutralizing antibodies—is relatively small, says Jason McLellan, a structural biologist at the University of Texas at Austin, and the region might be able to tolerate only so much change and still perform its main job of attaching itself to host cells’ ACE2 receptors.
It’s also possible that repeated exposure to different versions of spike—through infection with different virus strains, vaccine updates or both—could eventually build up a wall of immunity that SARS-CoV-2 will have difficulty overcoming. Mutations that overcome some people’s antibody responses are unlikely to foil responses across an entire population, and T-cell-mediated immunity, another arm of the immune response, seems to be more resilient to changes in the viral genome.
Such constraints might slow SARS-CoV-2’s evasion of immunity, but they are unlikely to stop it, says Bloom. There is clear evidence that some antibody-dodging mutations do not carry large evolutionary costs, says McLellan. “The virus will always be able to mutate parts of the spike.”
A virus in transition
How SARS-CoV-2 evolves in response to immunity has implications for its transition to an endemic virus. There wouldn’t be a steady baseline level of infections, says Kucharski. “A lot of people have a flat horizontal line in their head, which is not what endemic infections do.” Instead, the virus is likely to cause outbreaks and epidemics of varying size, like influenza and most other common respiratory infections do.
To predict what these outbreaks will look like, scientists are investigating how quickly a population becomes newly susceptible to infection, says Kucharski, and whether that happens mostly though viral evolution, waning immune responses, or the birth of new children without immunity to the virus. “My feeling is that small changes that open up a certain fraction of the previously exposed population to reinfection may be the most likely evolutionary trajectory,” says Rambaut.
The most hopeful—but probably least likely—future for SARS-CoV-2 would be to follow the path of measles. Infection or vaccination provides lifetime protection, and the virus circulates largely on the basis of new births. “Even a virus like measles, which has essentially no ability to evolve to evade immunity, is still around,” says Bloom.
A more likely, but still relatively hopeful, parallel for SARS-CoV-2 is a pathogen called respiratory syncytial virus (RSV). Most people get infected in their first two years of life. RSV is a leading cause of hospitalization of infants, but most childhood cases are mild. Waning immunity and viral evolution together allow new strains of RSV to sweep across the planet each year, infecting adults in large numbers, but with mild symptoms thanks to childhood exposure. If SARS-CoV-2 follows this path—aided by vaccines that provide strong protection against severe disease—“it becomes essentially a virus of kids,” says Rambaut.
Influenza offers another scenario—in fact two. The influenza A virus, which drives global seasonal influenza epidemics each year, is characterized by the rapid evolution and spread of new variants able to escape the immunity elicited by past strains. The result is seasonal epidemics, propelled largely by spread in adults, who can still develop severe symptoms. Flu jabs reduce disease severity and slow transmission, but influenza A’s fast evolution means the vaccines aren’t always well matched to circulating strains.
But if SARS-CoV-2 evolves to evade immunity more sluggishly, it might come to resemble influenza B. That virus’s slower rate of change, compared with influenza A, means that its transmission is driven largely by infections in children, who have less immunity than adults.
How quickly SARS-CoV-2 evolves in response to immunity will also determine whether—and how often—vaccines need to be updated. The current offerings will probably need to be updated at some point, says Bedford. In a preprint published in September, his team found signs that SARS-CoV-2 was evolving much faster than seasonal coronaviruses and even outpacing influenza A, whose major circulating form is called H3N2. Bedford expects SARS-CoV-2 to eventually slow down to a steadier state of change. “Whether it’s H3N2-like, where you need to update the vaccine every year or two, or where you need to update the vaccine every five years, or if it’s something worse, I don’t quite know,” he says.
Although other respiratory viruses, including seasonal coronaviruses such as 229E, offer several potential futures for SARS-CoV-2, the virus may go in a different direction entirely, say Rambaut and others. The sky-high circulation of the Delta variant and the rise of Omicron—aided by inequitable vaccine roll-outs to lower-income countries and minimal control measures in some wealthy countries such as the United States and the United Kingdom—offer fertile ground for SARS-CoV-2 to take additional surprising evolutionary leaps.
For instance, a document prepared by a UK government science advisory group in July raised the possibility that SARS-CoV-2 could become more severe or evade current vaccines by recombining with other coronaviruses. Continued circulation in animal reservoirs, such as mink or white-tailed deer, brings more potential for surprising changes, such as immune escape or heightened severity.
It may be that the future of SARS-CoV-2 is still in human hands. Vaccinating as many people as possible, while the jabs are still highly effective, could stop the virus from unlocking changes that drive a new wave. “There may be multiple directions that the virus can go in,” Rambaut says, “and the virus hasn’t committed.”
This article is reproduced with permission and was first published on December 7 2021.