Last summer, as the second wave of COVID-19 cases was sweeping the United Kingdom, a man in his 70s was admitted to his local hospital where he tested positive for SARS-CoV-2. He was sent home, but a month later he checked into the hospital at Cambridge University, unable to shake the virus. Like many people who develop severe COVID-19, the man was immunocompromised. He had lymphoma and had previously received chemotherapy treatment.
Doctors gave him remdesivir, an antiviral drug used to treat COVID-19, but he showed little improvement. Two months after his illness began, as the patient continued to worsen, his medical team opted to treat him with convalescent plasma, a therapy derived from the blood of patients who have recovered from COVID-19, which contains antibodies to fight off the virus.
Sadly, he succumbed to the virus 102 days after testing positive, but what doctors learned from him and similar patients “has been transformative of our understanding of what’s going on in this disease,” says Ravindra Gupta, a member of the Cambridge Universitymedical team and senior author of a report of the man’s case published February 5 in Nature. Analysis of samples from the patient showed that the virus evolved rapidly after the plasma therapy, developing mutations that changed how it could infect cells and resist antibodies. The conditions turned out to be ripe for viral evolution. “This is a blueprint for how variants emerge,” Gupta says.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The plasma treatments did not rid the man of the virus, and in fact had little impact on the amount of virus detected. But the plasma had a remarkable effect on the genetic makeup of the viral population that the patient harbored. Seemingly in response to the antibodies contained within the plasma, the virus produced “escape mutations,” changes in the genetic code that helped it to evade detection by the sticky antibodies. Such mutations can make the virus more contagious or vaccines less effective. They are popping up in variants of SARS-CoV-2 around the world, fueling the pandemic even as vaccine shots are going into people’s arms.
Over the several months of the patient’s treatment, doctors collected samples of the virus and determined their genetic sequences. The infection started out as a genetically singular population, but it underwent subtle changes after treatment with the antiviral drug remdesivir. “And then things really changed when we tried convalescent plasma,” Gupta says.
Random changes in any viral genetic sequence are normal over the course of an infection, but a striking pattern emerged in Gupta’s patient. After the plasma infusions, viruses containing multiple new mutations appeared and quickly dominated, but not for long. Two weeks later, when antibody levels were expected to have diminished, the mutant virus populationvanished. But then the patient received a final plasma treatment. Remarkably, the mutant strain came roaring back. Gupta’s team conjectured that the genetic changes appear to have occurred in response to the plasma treatment. This phenomenon, called selective pressure, may have occurred when viruses with mutations resistant to the antibodies survived.
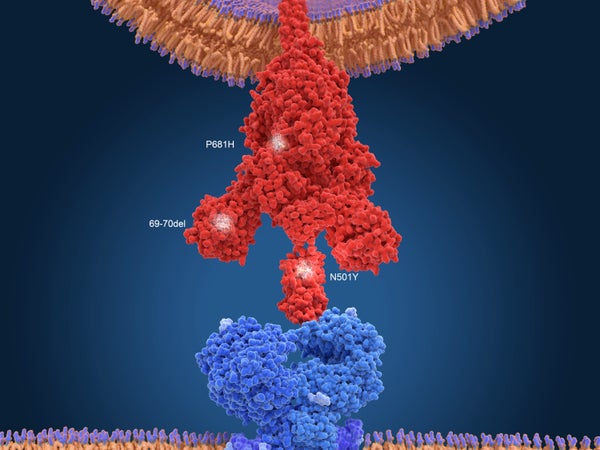
The post-plasma virus strain carried two particular mutations: one, called D796H, has been only rarely documented before. The other, resulting in the deletion of two amino acids, called ∆H69/∆V70, “has gained notoriety, because it’s one of the defining mutations in the B.1.1.7 lineage,” says Gupta, referring to a strain originally found in the U.K., which has been shown to be more transmissible. (Gupta believes that the B.1.1.7 strain likely emerged from an immunocompromised patient, but the data show it did not originate in this specific patient.)
To explore the impact of these two mutations, the researchers introduced them—both together and separately—into SARS-CoV-2 cultured in a lab dish. Viruses with the D796H mutation or with both mutations were better at evading antibodies, suggesting that antibodies exerted “pressure” to generate mutations. The D796H mutation alone made the virus worse at infecting cells. The ∆H69/∆V70 deletion alone, however, made the virus twice as efficient at infecting cells—a hallmark of B.1.1.7. Virus with both mutations infected cells similarly to the original unmutated population, suggesting the two mutations canceled one another out when it came to infectiousness. The analysis showed that the D796H mutation arose first, and Gupta postulates that the deletion may have arisen in response. “It’s as if the virus was trying to fix itself,” he says, by making up for the infectivity deficit.
Rapid evolution of the virus has been documented in other patients as well, including a Boston man hospitalized at Brigham and Women’s Hospital, which was reported December 3 in the New England Journal of Medicine. Jonathan Li, a virologist and an author of that case study, says his group also found multiple mutations, but they didn’t understand the implications until later. Once those same mutations were described in B.1.1.7 and other variants and were found to change the virus’s behavior, “that’s when we realized [it] was a harbinger of what’s to come.”
It’s impossible to know how many people are carrying around an actively evolving virus. Perhaps most worryingly, another case reported November 4 in Cell demonstrated such viral evolution in an asymptomatic patient over several months. “We need to figure out exactly how and when this is happening, in whom and at what frequency, and what to do about it,” Gupta says. Immunocompromised patients are by no means the only source of new mutant variants, Li says. "Viruses mutate in immunocompetent people, too, especially in the setting of unchecked spread and [viral] replication.
Meanwhile, Li says, the findings have immediate, real-world implications. “We need to pay a lot more attention to immunocompromised patients, and we have to be careful, to make sure they’re eventually able to clear their virus. We need to care for them in an intensive way.” The rules around isolation might also need to account for such patients. “The CDC criteria say that you can leave isolation 10 days after the onset of symptoms, and that’s probably fine for the vast majority of people,” but immunocompromised people may need to isolate for longer, Li says.
The bottom line, the virologists agree, is that in the wake of these new variants, widespread vaccination has become even more urgent. Until then, you know the drill: mask up and stay physically distanced.
Read more about the coronavirus outbreak from Scientific American here. And read coverage from our international network of magazines here.