Key concepts
Chemistry
Gases
Acids
Reactions
Combustion
Introduction
Do you have a fire extinguisher at home? Hopefully, yes! It can save your life in case there is a fire. But have you ever wondered how these extinguishers work? Some of them don’t even contain water, which is what you probably think of first when it comes to stopping a fire from spreading. What else other than water can extinguish a flame? Do this activity to find out—and put out a candle as if by magic!
Background
What actually happens when something is burning? A fire, also called combustion, is the result of a chemical reaction in which oxygen gas reacts with the substance that is burnt. Luckily, not everything catches fire right away when exposed to air or oxygen. For a fire to start, certain requirements have to be met: First, you need a combustible material, or a fuel, that can burn. Second, this material needs to be hot enough so that it can catch fire. The combustion reaction then has to create enough heat to sustain the fire. And lastly, a fire needs oxygen to burn. If either heat, fuel or oxygen are absent, there will be no fire.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Fire extinguishers are designed to remove one of the necessary “ingredients” for a fire to burn. There are several ways this can be done. For example, water can be used to remove heat from a fire. Adding water often cools down the fuel enough so that it stops burning. Some types of fire extinguishers use dry chemicals to interrupt the chemical combustion reaction. Yet another option is to cover the fire with a layer of foam, carbon dioxide gas or dry chemicals that stop air from reaching the flames. Each of the aforementioned methods results in the same outcome: the fire is extinguished. You can see for yourself in this activity, and you don’t even have to use a real fire extinguisher—just some baking soda and vinegar. Curious about how these two ingredients can put out a flame? Then go ahead and try it out!
Materials
Small candle
Matches
Adult helper
Vinegar
Baking soda
Tablespoon
Play-Doh or modeling clay
Glass dish in which to place your candle (The candle should not be too tall; its must be lower than the top of the dish.)
Water
A safe area in which to perform this activity
Preparation
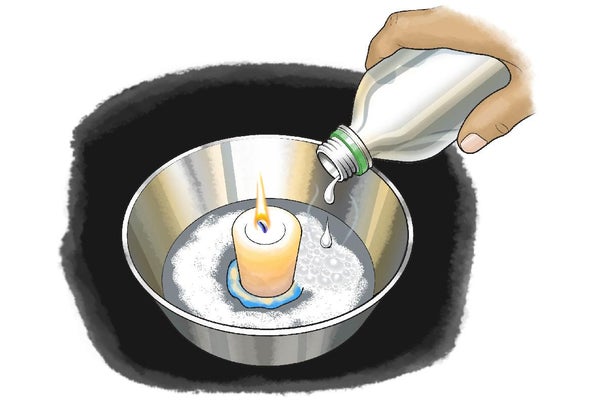
Use the Play-Doh or modeling clay to stick the candle into the center of your glass dish.
Sprinkle a couple of tablespoons of baking soda around the candle. The bottom of the dish should be fully covered with about a quarter inch of baking soda.
Procedure
With the help of an adult, light the candle with a match. Observe the flame for a couple of seconds. What does the flame look like? Is it big or small?
Carefully pour some vinegar onto the baking soda in the glass dish. It should be enough to make all the baking soda dissolve. Make sure, however, that no liquid or foam reaches the flame. What happens when you add the vinegar to the baking soda? Can you explain your observations?
Once you have added the vinegar, watch the candle carefully. What happens to the flame? Does the candle keep burning? Note: If you do not see anything happening, try to increase the amount of baking soda or vinegar that you add to your glass dish.
Try to light the candle with a match again if the flame went out. Is it easy or hard to do that? What happens to the match if you get close to the candle?
Clean out your glass dish and repeat the experiment again, but this time instead of vinegar, pour water on top of the baking soda. What happens to the flame this time? Do you observe the same reaction? If not, can you explain the difference?
Extra: Can you find other liquids in your kitchen beside vinegar that make carbon dioxide when mixed with baking powder? Remember, these liquids have to be acidic. Observe your burning candle when you add these solutions to the baking soda. How do they react? Does your flame keep burning or is it extinguished?
Extra: Together with an adult, locate a fire extinguisher in your house (if you have any) and read its ingredients list. Can you find out how it works to extinguish a fire? Ask an adult about how to use this extinguisher in case of an emergency. But be careful to not accidently use it for real!
Observations and results
Did you successfully extinguish your flame? You don’t even see what makes the candle go out, but it does—almost looks like magic! Initially, your candle should have burned bright and steady. After all, it had enough fuel from the candle, heat from the match and oxygen from the air to sustain a fire. You should have observed, however, that after you added the vinegar to the baking soda in the glass dish, a short time later the flame suddenly went out. What happened? The answer has to do with the chemical reaction that occurs when you mix vinegar with baking soda.
Baking soda is another name for the chemical compound sodium bicarbonate. It reacts with any type of acid such as vinegar to form gaseous carbon dioxide (CO2). You can actually see it being produced—it makes the bubbles and foam that you observe when you add the vinegar to the glass dish. The same carbon dioxide reaction makes a baking soda volcano erupt or inflates a balloon when you mix an acid with sodium bicarbonate. In this experiment the CO2 extinguishes the candle flame. Although you can’t see it, the CO2 produced by the baking soda–vinegar reaction starts filling the glass dish from the bottom up. Eventually, once all of the air in the glass dish is replaced by carbon dioxide, the flame has no more oxygen left to burn and it goes out. If you try to light the candle again, it won’t ignite because the match also goes out once it enters the carbon dioxide layer in the glass dish.
When you add just water to the baking powder in the glass dish, however, no carbon dioxide is produced because water is not an acid. Therefore, the candle should have kept burning as long as enough oxygen and fuel was available.
This activity was inspired by 101 Great Science Experiments, from Neil Ardley.
Cleanup
Make sure you put out the candle. Rinse out the glass dish with water and soap, and discard the Play-Doh or modeling clay.
More to explore
What Is Fire?, from Science Learning Hub
How Fire Extinguishers Work, from HowStuffWorks
Spectacle Science: Exploring Homemade Rockets, from Scientific American
Science Activity for All Ages!, from Science Buddies
This activity brought to you in partnership with Science Buddies