It was the middle of the night in Jerusalem, and we were watching mice swim. The year was 1994, and the two of us were crouching over a pool of cold water in a laboratory at the Hebrew University. The room was chilly, our hunched backs ached, and we had been repeating this routine over many nights, so we were tired and uncomfortable. So were the mice. Mice really dislike swimming, especially in cold water—but we wanted to stress them out.
We humans were on the night shift because both of us had other things to do during the day. Kaufer was working on a doctorate in molecular neurobiology, and Friedman was an Israel Defense Forces physician and was often on call. What brought us together with the mice every evening was an attempt to understand a medical mystery: Gulf War syndrome. After the conflict ended in 1991, there were an increasing number of reports of soldiers from the U.S.-led coalition who were afflicted with chronic fatigue, muscle pain, sleep problems and cognitive deterioration, and those soldiers were hospitalized at higher rates than nondeployed veterans. Some doctors suspected that pyridostigmine, a drug that had been given to soldiers to protect them from chemical weapons, could cause these ailments if it made it into their brains.
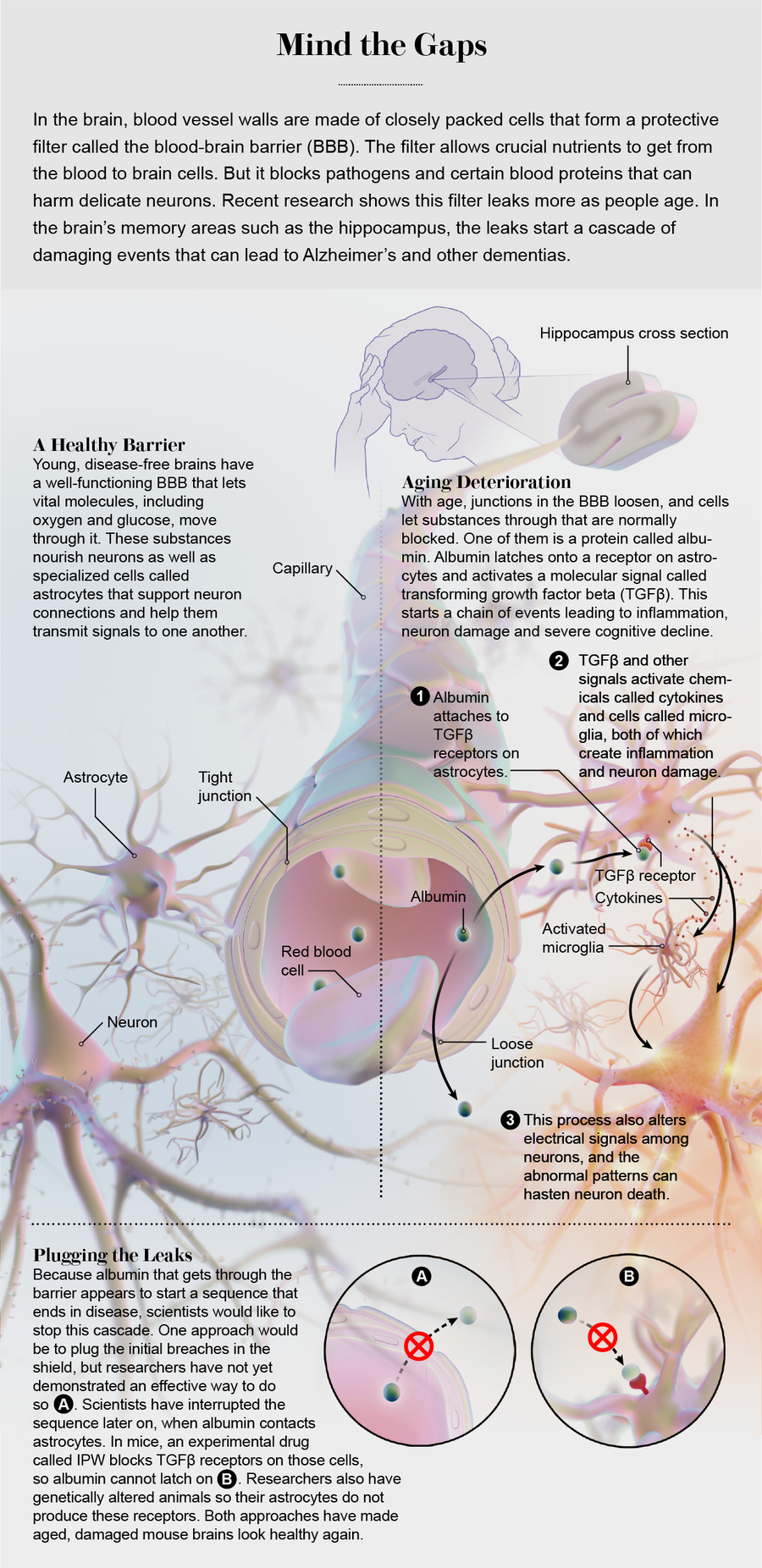
There was a big problem with this theory, however: pyridostigmine in the bloodstream was not supposed to reach the brain. Blood vessels that course through this vital organ have walls made of specialized cells, packed very closely and with abilities to selectively control what can get into the brain. They form a shield that keeps toxins, pathogens such as bacteria and viruses, and most drugs safely within the vessels. This structure is called the blood-brain barrier, or BBB for short, and the drug should not have been able to pass through it.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
That is, if the barrier was intact. We were testing the hypothesis that the physical and mental stress of combat might somehow trigger leaks in the shield. The swimming mice were our way of testing whether stress led to damage. When the swim session was done, we pulled each mouse from the pool and injected a drop of blue dye into one of its veins. Then we waited as the dye passed through its body, gradually turning the mouse blue. If the BBB was intact, the brain should have remained its normal pinkish-white color. We euthanized the mice so we could take a look at their brains under a dissecting microscope. Over several nights we had tried various lengths of swim time, but we did not see any brain changes.
But on this night, after two dips in slightly colder water, things looked different: the brains had a strong blue tint! Lab work is usually tedious, and success is often subtle, but this time we were jumping up and down and hugging each other, giddy with excitement. Our weird experiment had worked. Stressful situations could make the BBB spring leaks. With the help of our mentor, neuroscientist Hermona Soreq, we went on to show that the effect occurred with pyridostigmine and altered brain cell activity. We published these results in 1996 in Nature Medicine and in 1998 in Nature.
A quarter of a century later we can say that looking at these blue brains turned out to be a defining moment for both our careers, as well as the beginning of a lifelong friendship and scientific collaboration. Discovering the telltale blue tinge was the first step on a path that, over many years, led us to probe more and more deeply into the connection between other brain diseases and flaws in the organ's protective shell. Today pyridostigmine penetration is a leading hypothesis for the cause of Gulf War syndrome, although there are other candidates. And our investigations have linked BBB damage—caused by aging in addition to injury or acute stress—to several more familiar illnesses: Alzheimer's and related dementias, epilepsy and traumatic brain injury. In two papers published in 2019 in Science Translational Medicine, we demonstrated that as people get older, this shield loses integrity and starts leaking, allowing blood proteins into the brain that normally do not get there. These proteins in turn activate a cascade of events among brain cells that can produce some of the most notable and widely seen changes associated with aging and illness: inflammation, abnormal neuron activity and cognitive impairment.
The cause-and-effect connection looks especially strong because stopping the reactions set off by these leaks actually reverses signs of disease, at least in rodents. In older mice, we can abolish the inflammatory fog with a targeted drug that protects brain cells from being irritated by blood proteins or by making genetic modifications that prevent those cells from releasing inflammatory molecules. Within days of the treatment the aged brains of these mice started to function more like young brains. Abnormal electrical activity subsided. Markers of inflammation dropped to low levels. When placed in mazes, the animals made their way through as quickly and accurately as young mice did. We cannot try the same experimental brain modifications in humans; it is not ethical. But we have been able to use magnetic resonance imaging techniques, electroencephalography recordings and analysis of postmortem brain specimens to compare the brains of aged people and of individuals with Alzheimer's with those of young and healthy people. The images show excessive and progressive BBB disruption and leaking with aging and in those with the disease, as well as other features of the chemical cascade.
We do not know whether a damaged barrier is the only cause of Alzheimer's or other brain illnesses. It could play a contributing role along with other causes, including genetics and a variety of cellular problems that have been seen in aging brains. Or it could be collateral damage. And experiments in mice often do not pan out in people. But right now the long-standing dominant theory for Alzheimer's—that it is triggered by a buildup of a protein called beta-amyloid in the brain—is looking less convincing than ever. Many people have high levels of beta-amyloid in their brain but show no decline in mental function. Moreover, numerous experiments have reduced levels of this protein in the brain, yet the disease and associated mental decline remained unaffected. Drugs that target beta-amyloid have failed to help. Given that there are now more than 55 million people worldwide with dementia and almost 10 million new cases diagnosed every year, according to the World Health Organization, many scientists say it is high time to consider alternative explanations and therapeutic directions. If flaws in the brain's protective shield start a chain of events that leads to disease—a chain that experiments suggest can be blocked to restore brain health—it is a path of investigation worth pursuing.
Gaps in the Wall
With “barrier” in its name, the BBB sounds like a wall around the brain, but it is really more like a distributed filter. Our body's control center gets 20 percent of the oxygen-rich blood pumped out by the heart, delivered by an intricate mesh of blood vessels. They look different from vessels in the rest of the body, with walls made of tightly packed cells with particular transport systems that form a semipermeable filter. Networks of brain cells need a carefully controlled environment to function, so this filter lets molecules such as oxygen and glucose diffuse through but blocks blood proteins, certain ions, immune system cells and pathogens. This protective mesh extends throughout most areas of the brain, from the outer layers of the cortex, where higher-order cognition occurs, to deep places such as the hippocampus, which regulates memory storage. Problems with the filter can therefore lead to all kinds of neurological difficulties.
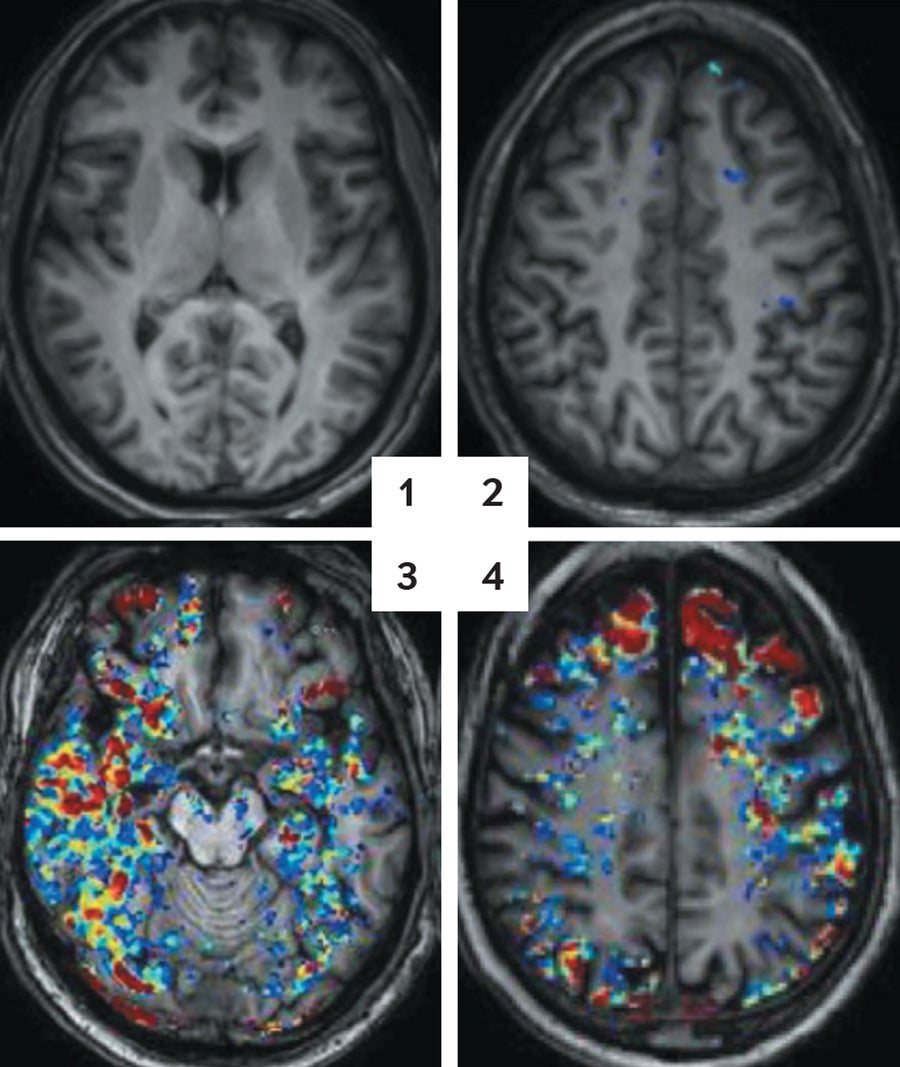
AGING BARRICADE: Brain scans, highlighting a colored tracer molecule in the blood, show more leaks in the blood-brain barrier as people age. A 30-year-old looks clear (1). At age 42, blue spots indicate small seeps (2). By age 65, red and yellow spots show bigger flows (3). At age 76, the pattern continues (4). Credit: Alon Friendman and Daniela Kaufer
Back in the 1990s, as we were completing our initial work on Gulf War syndrome, we knew that other researchers had noted BBB damage in patients with some brain disorders, including Alzheimer's. But we did not know whether this problem was a cause or an effect or how leaks in the shield get started and what they might do to alter brain function. We did, however, want to find out.
After our time working in Jerusalem, Kaufer went to Stanford University for her postdoctoral fellowship, and Friedman continued his medical training in Israel, specializing in neurosurgery. But time and distance did not let us forget. On a vacation together with our families, sailing between Greek islands, we caught up. Kaufer was learning more about how stress affects the brains of mice in work at Stanford. Friedman, in his own practice, was reaffirming the early observations from other researchers who saw flawed BBBs in many patients suffering from very different neurological conditions. Just what was the damaged barrier doing?
We began to figure out the answer to this question in the mid-2000s, when Friedman got the chance to work in Berlin with the late neuroscientist Uwe Heinemann of the Institute for Neurophysiology, part of the Charité Center for Basic Medicine. Heinemann opened his lab to the next key experiment. We wanted to observe brain function directly after the BBB started to malfunction, so we gave rats a chemical that essentially poked holes in the barrier and then dissected their brains at various later times. We kept the brain slices alive in nourishing fluid and used an electrode to record the electrical signals that the cells used to communicate with one another in the presence of a leaky barrier.
The first few days were boring. The neurons were giving off signals one after another in staccato, irregular patterns, “talking” as if nothing unusual had happened. We almost decided to give up. Then, on the fifth day, the cells' chatter patterns changed. More and more neurons started to pulse together in synchrony. After a full week we nudged them with a small signal from an electrode, mimicking a brief electrical message within the cerebral cortex. This nudge produced a storm of cells firing together, similar to what is observed in people and animals with epilepsy.
We think what happened with these cells is analogous to generating a Twitter storm. Imagine that you created a Twitter account today and tweeted some sensational statement. You would probably get a very small response because you would not have many followers. If in the next few days you built a bigger network of followers and tweeted again, however, the same statement would be likely to be retweeted, recruiting more followers who would also retweet it, eventually leading to a storm of tweets on the social media platform. Similarly, when we disrupted the BBB, neurons in the brain were not discombobulated right away, but after they had spent a week building a new network of connections, a small jolt prompted a bigger electrical response. We now have recorded these electrical responses in the brains of people diagnosed with Alzheimer's and with epilepsy, and we termed those events “paroxysmal slow-wave events.”
This storm happened only after we mimicked a BBB leak. Without one, our brain slices were untroubled by any electrical tempests. So we hypothesized that there was some element from the blood that was reaching these neurons to trigger the brain reaction. We tested this theory in a young, healthy rat with a normal BBB by injecting blood directly into its brain—bypassing the barrier—and monitoring electrical activity. It took several days, but again the storm built and exploded. Clearly, it had something to do with the blood. But blood is a complex fluid containing many different kinds of cells and proteins, so we set off on a painstaking filter-and-trap expedition. We used various filters to capture blood components of different sizes, then repeated the injection to see whether the storm recurred. It was a process of elimination. Eventually we found one blood protein that created the disturbances: albumin.
The Start of Trouble
We were not thrilled with our catch. Albumin is very common and is involved in many bodily functions, so it was hard to isolate what it was doing in this situation. We would have preferred a rarer component. But albumin was what we got, so we dug in. Kaufer moved to the University of California, Berkeley, to run her own lab, and Friedman started his, first at Ben-Gurion University of the Negev in Israel and later at Dalhousie University in Nova Scotia. We planned a joint, long-distance series of experiments over several years to delineate the steps from BBB disruption and albumin leakage to the appearance of neurological disorders.
The first thing we learned was that when albumin gets into the brain, it appears to stimulate astrocytes, key brain cells that provide structural, functional and chemical support for neurons and their connections. When albumin contacts an astrocyte, it binds to receptors on the cell that under normal conditions bind a signaling molecule called transforming growth factor beta (TGFβ). Among other things, TGFβ provokes inflammation, starting a cascade of molecules called cytokines, and activates astrocytes and sentinel cells called microglia. Normally, this mechanism is the brain's way of limiting damage by destroying malfunctioning cells in a targeted assault. But when albumin seeps in, it leads to a positive-feedback loop of overproduction of TGFβ and other cytokines, setting in motion a chain of events. Recent evidence describes “senescent” cells—cells that remain chemically active after their cell-proliferation functions have stopped—that detect tissue damage and turn on a genetic program that amplifies the signal, broadcasting it to neighboring cells. We showed in a paper published this year in Aging Cell that albumin that seeps into the brain induces astrocytes to become senescent. Eventually lots of brain cells get damaged, key neural connections are modified and the function of these circuits deteriorates.
Credit: Now Medical Studios
These findings tracked the steps from a leak in the BBB (induced by brain injury, for example) to neural dysfunction and the possible development of epilepsy. We became curious about whether this offered a possible explanation for why brain function deteriorates with age in some individuals, and indeed, subsequent experiments showed how this sequence of events plays out in aging mice. The animals typically live a bit more than two years on average. We allowed a colony of mice to age peacefully and looked inside their brains at various points. Albumin, we saw, was not in the brain at all in younger mice, but it began to show up in middle age. The effect was modest at first, but there was a clear decline in the integrity of the barrier, and it got worse in some mice as they got older. The affected mice also had more trouble learning and remembering their way through mazes than did their younger and relatively albumin-free counterparts.
When the albumin showed up, other experiments revealed, TGFβ receptors started to get active. We stained the mouse brains with antibodies that recognized an inflammatory protein by-product of TGFβ-receptor activation, and then we used green fluorescence to localize astrocytes that had albumin and TGFβ-receptor hyperactivity. The inflammatory signal always started after albumin appeared in astrocytes and increased with a greater degree of albumin leakage. We saw that albumin and the inflammation it caused were especially abundant in the hippocampus, a brain area that is a key component in memory regulation.
Within the past decade we have been able to provide good evidence that this same process happens in people. We used tracer molecules to tag signals of barrier leakage in people. With MRI, we could see how the brain changes its signal when the tracer shows up minutes after being injected. Similar to mice, some middle-aged people already showed the tracer leaking into their brain at that point, and the older people were, the leakier their barrier became. Other researchers, such as Berislav V. Zlokovic of the University of Southern California Keck School of Medicine and his colleagues, used slightly different imaging methods to show similar age-related deterioration in barrier integrity in the hippocampi of living people with cognitive impairment. In our work, we added autopsies of a separate group of people and showed that heightened albumin levels accompanied greater amounts of TGFβ, always in astrocytes. These concentrations were higher in older people and in individuals who had died of Alzheimer's than in those without the disease.
Brain Rejuvenation
Now we knew that there was a correlation between BBB dysfunction in aging mice and the process in humans. But demonstrating correlation does not prove causation. First, we proved that this mechanism is sufficient to activate an aging program in the brain: When we infused albumin into the intact healthy brains of young mice for one week, these brains functioned like aged brains, with abnormal neuronal function, higher susceptibility to seizures and reduced learning capacity (the mice had a hard time learning an escape route in a water maze task). Furthermore, activation of this mechanism was essential for the aging response: we reversed the deterioration in mice by blocking the albumin-TGFβ cascade that came after the leaks. We developed a group of mice in which we genetically cut out the portion of DNA that tells astrocytes to produce TGFβ receptors, eliminating that feature from the cells. When the mice were still relatively young, we implanted a tiny pump in their brains that injected albumin. We did the same thing to a group of young, normal mice. Then we put both groups into a tricky water maze. (Watching mice swim seems to be a recurring theme with us.) The mice with receptors (and albumin in their brains) had a lot of trouble. But the animals without receptors swam the maze like young, healthy mice—speedily and accurately—and when we changed the maze configuration, they learned the new route, too. When we looked at their brains, we saw low levels of inflammation, senescent astrocytes and abnormal electrical activity.
This was really very encouraging. But for people, the option of knocking out a gene for a brain feature will not be an available therapy any day soon. There is, however, another form of treatment. Barry Hart, a medicinal chemist at Innovation Pathways, a start-up drug company in Palo Alto, Calif., had designed an anticancer drug that specifically blocked the activity of the TGFβ receptor. Such growth factors play a role in tumor progression, so blocking them could be therapeutic. Hart contacted us and suggested that we try the drug, called IPW, on our mice.
When we gave the drug to middle-aged mice—the ones that were starting to show albumin leakage—we learned that it made their brains look young again. TGFβ activity dropped to levels seen in youthful mice, markers of inflammation went way down, and abnormal electrical activity and seizure susceptibility diminished.
But the big surprise came when we tested actual behavior and cognition. We set up another maze, and this time we ran older mice through it. Some of the aged animals were treated with IPW, and some were not. We did not predict a lot of improvement, because we thought irreversible damage had already been done. (Our mice without the TGFβ gene had been spared the long months of deterioration inflicted by the inflammatory cascade, but these animals had not.) Within days, however, the treated mice were almost as good at learning the maze as rodents half their age. The untreated mice just shambled along as usual. Moreover, the mice that got IPW showed no sign of the “Twitter storm” effect that we typically see in humans with Alzheimer's or epilepsy and not much evidence of inflammation. It was as if an inflammatory fog had lifted, allowing the brain to regain its youthful abilities. These, along with the studies of human brains, are the results we published in two papers in 2019 in Science Translational Medicine.
The maze outcome was so unexpected, even to us, because, like most people, we had considered aging damage as a one-way trip—deterioration that cannot be undone. That is probably the case for major brain trouble, such as the havoc that occurs in individuals with Parkinson's disease or in advanced Alzheimer's once clumps of beta-amyloid have accumulated to such a degree that they kill off swaths of neurons and other cells. But this work may indicate that in the absence of a lot of cell death, the aging brain has a hidden capacity to rebound from some types of insults.
And our findings have implications for acute injuries as well, not just gradual deterioration. Treating rodents with IPW after concussions or traumatic brain injury with this drug alleviated the inflammation, seizures and cognitive decline that developed in the placebo-treated animals.
Fixing the Damage
The world population is aging, and the number of people with dementia and Alzheimer's is on the rise. Neuroscientists have a poor understanding of the early triggers of the transition from a young, healthy brain to an old, dysfunctional one. Alzheimer's and other neurological diseases of aging are complex. Defects in the way the brain disposes of aberrant proteins may play a role in how these illnesses get started, or the trigger could be the impairment of electrical signals among neurons, to name just two possibilities.
Now a leaky BBB has to be considered a strong contender as well, although it might not be the only cause or the only route to treatment. This barrier-breach theory provides a remarkably intuitive and straightforward new model to understand why the brain declines with age. And it is a model that gives us optimism: the results of our work strongly hint that the aging brain retains a capacity for reshaping and restoring itself, an ability that may be chronically suppressed, but not irreversibly lost, by persistent leakiness and the ensuing chain of events we have traced.
Our next step is to look for strategies and therapies to reduce barrier leakage. In the past, pharmaceutical research into the barrier focused on ways to increase permeability, not limit it, to get more drugs across it to treat brain tumors or infections. Our results show that it is time to flip the question: Can we come up with ways to stop the shield from degrading, stop harmful substances from getting across, or at least interrupt the fall of molecular dominoes if they do? There is a chance to do a lot of good for a lot of people if we can figure these things out.