Sarah Vitak: This is Scientific American’s 60 Second Science. I’m Sarah Vitak.
So many of the objects we interact with nowadays run on programming and are designed for an exact purpose. We don’t typically think of living things as falling into this category, but more and more scientists are programming and designing living cells and even whole organisms.
This area of research is called synthetic biology, and there is a whole subsection of this field that is all about figuring out how to engineer cellular armies that can be commanded and controlled: cells trained to search out diseased tissues; cells equipped for environmental reconnaissance; cell assassins that put out hits on other cells.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Sounds cool, right? But in reality, nothing is that targeted and exact.
Mikhail Shapiro: It’s somewhat limited, because those cells, to date, don’t really have spatial awareness, they don’t know, spatially, where they are in the body: Are they in a tumor? Are they in the liver? Are they in the pancreas? Are they in the brain? So, you know, they might try to figure that out based on molecular clues. But they don’t have this kind of GPS that’s telling them that they’re in the right place to carry out their actions.
Vitak: That is Dr. Mikhail Shapiro, a professor of chemical engineering at Caltech. His lab recently tackled this problem head on using the power of air bubbles. [Avinoam Bar-Zion et al., Acoustically triggered mechanotherapy using genetically encoded gas vesicles]
Their lab had already been doing a lot of work with programming cells to create tiny structures called gas vesicles. They are basically just little air bubbles surrounded by a hard shell of protein that then float around inside the cell. You can think of them like nanoscale Ping-Pong balls.
Shapiro: They have a fascinating origin. They basically evolved as flotation devices for photosynthetic microbes that live in water and need to float to the top of the water so they can get enough sunlight.
Vitak: Dr. Shapiro and his team had previously been able to put the genes for making gas vesicles into mammalian cells or other bacteria. At the time, they were doing this because they wanted to use it as a tool for imaging.
They were inspired by jellyfish—more specifically, something that they make called green fluorescent protein, or GFP. The good thing about this protein is: it can be genetically encoded to be attached to naturally occurring proteins, allowing scientists to view them and track where they go in living cells or animals.
Using GFP as an imaging tool totally changed the game in microscopy, so much so that its discovery and development was awarded a Nobel Prize in 2008.
Shapiro: So we were kind of obsessed about finding something similar to a fluorescent protein but that, instead of being fluorescent, meaning interacting with light, would interact with sound waves and allow us to image it with ultrasound. And sound waves get scattered, or reflected, off of materials that have a different density, or stiffness, relative to their surroundings. And air, you know, is very different in its density and its compressibility, sort of, compared to tissue and water, and so on.
Vitak: But recently they had the idea that maybe they could use these vesicles for other purposes—namely, by using ultrasound to intentionally burst the bubbles.
Shapiro: If we just crank up the ultrasound a little bit, then we’re hitting that Ping-Pong ball hard enough that it cracks and opens.
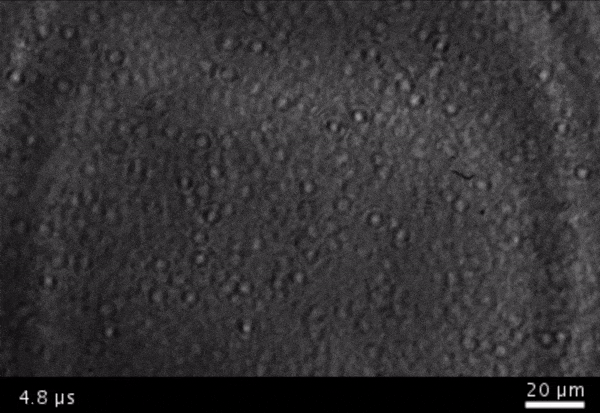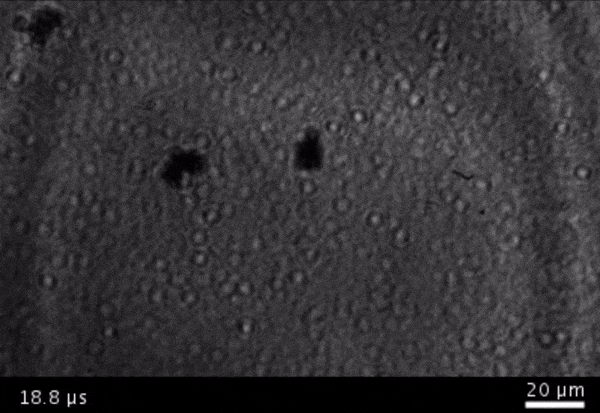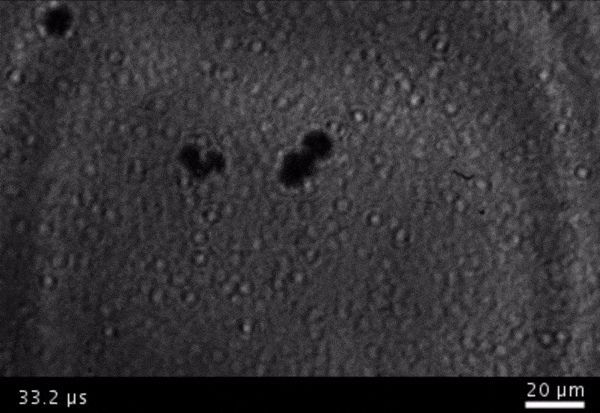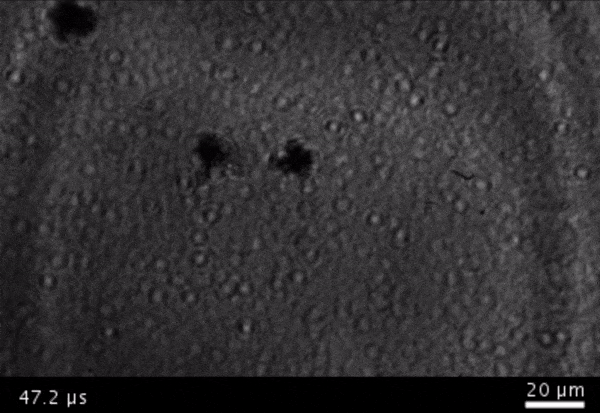
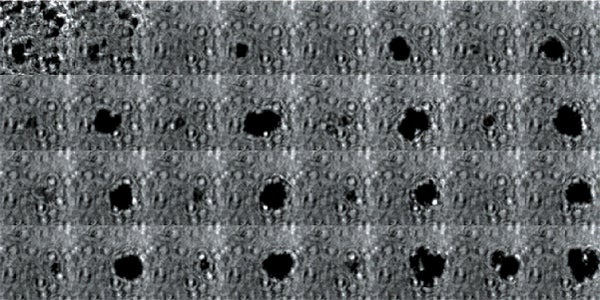
High-frame-rate movie of gas vesicles attached to a Mylar plate and shot at five million frames per second. The speed of the video has been significantly slowed down. Credit: Avinoam Bar-Zion
Vitak: When it does, the air is released, and so you have a little bubble inside the cell. Alone, it wouldn’t do anything. But as the ultrasound continues, the bubbles expand and contract.
Shapiro: And over time, several of these bubbles of air will come together and join up to form a larger bubble until it’s big enough that the ultrasound causes it to undergo a strong mechanical implosion.
Vitak: They started by getting this to work on the gas vesicles alone. They pulled a bunch of gas vesicles out of cells (an easy procedure because they just float to the top) and hit them with ultrasound. Then they listened in with an ultrasound mic and, sure enough, they could pick up the vesicles popping.
Shapiro: It’s ultrasound, so humans can’t hear it. But the spectrum of it looks like broadband noise. So when you turn on your TV, and it’s showing this like ...
[CLIP: White noise]
Shaprio: And I think that’s you would expect to hear if it were in the audible range.
Vitak: They wanted to see it as well.
Shapiro: So we borrowed a five-million-frame-per-second camera from our aeronautics department and connected it to a microscope ...
Vitak: ... which sounds excessive, but actually it wasn’t. Sure enough, they could see the vesicles breaking and forming the bubbles, which would then pop. And all of this would happen in a matter of microseconds.
Next they wanted to try to apply it, so they made gas vesicles that had proteins on the surface that would bind to cancer cells. Then they mixed them with cancer cells in a dish and hit it with ultrasound.
It worked.
Shapiro: Think of it as you’re punching the cancer cells. So these bubbles are expanding and contracting, and they’re punching and sending shockwaves very locally. And so that was pretty cool because, you know, we kind of converted our innocent little imaging agent into a little bomb that we put on a cancer cell and blow it up. But we thought it would be even cooler if we could get cells to be the bomb and have those cells go into the body and infiltrate a tumor to get inside.
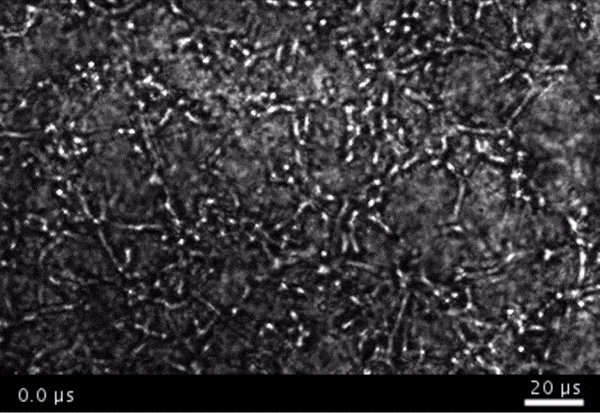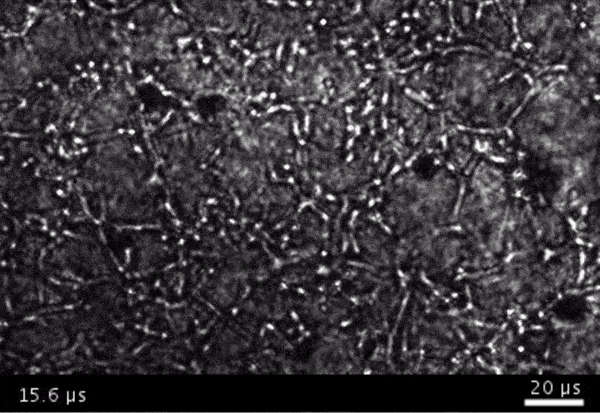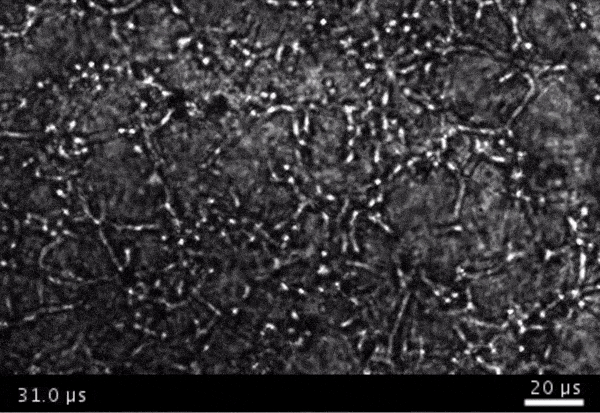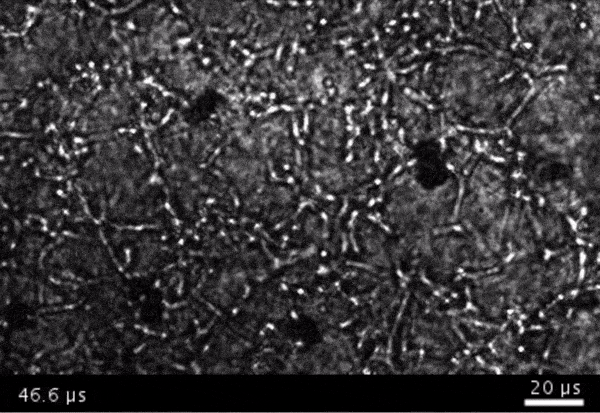
High-frame-rate movie of gas vesicles attached to cancer cells and shot at five million frames per second. The speed of the video has been significantly slowed down. Credit: Avinoam Bar-Zion
Vitak: So they tried the same experiments again, but this time they used bacteria that were engineered not only to make gas vesicles but also to home in on tumors.
In the petri dish, it worked.
Their next step was to try it inside an animal. This is where the ultrasound aspect really pays off because ultrasound can be used and targeted to a very specific location in the body. It basically makes up for that lack of cellular GPS I mentioned earlier.
So they injected some of these bacteria into the bloodstream of mice with tumors. And they saw ...
Shapiro: ... the bacterial cells kind of go different places around the body. But in most of the body, they get eliminated by the immune system—except in the core of the tumor because the tumor is an immunosuppressive environment.
Vitak: So basically using one of cancer’s key traits—and adaptations against it.
Shapiro: And what’s cool is that because the gas vesicles can serve as both an imaging agents and now a therapeutic agent. We could see, with ultrasound imaging, that the cells were in the tumor and could confirm that our little therapeutic agent got there and established a little colony inside the tumor center.
Vitak: Then they targeted ultrasound at the tumor. They did this in combination with a new class of cancer drugs that allow immune cells to find and kill cancer cells, known as immune checkpoint inhibitors. On average, the mice who received the bacteria and ultrasound treatment lived twice as long as the mice that only got the new cancer drugs.
This is maybe just the beginning for what we can do with these little targeted missile bacteria. They might also be promising for other disease treatments. Especially because you can have the bacteria carry a payload like a drug and then burst them open to release it in a particular location.
Shapiro: It’s a great example of Mother Nature giving us this, you know, precious gift. And it’s something that actually was discovered more than 100 years ago: the first paper noticing that there are these air-filled pockets in bacteria was published in 1895.
So I just think it's a neat story of going all the way from, you know, a couple billion years of evolution to then 100 years of basic biologists, you know, poking around at them—and now these proteins finding a whole new life.
Vitak: Thanks for listening. For Scientific American’s 60 Second Science, I’m Sarah Vitak.
[The above text is a transcript of this podcast.]